What is supercritical CO₂?
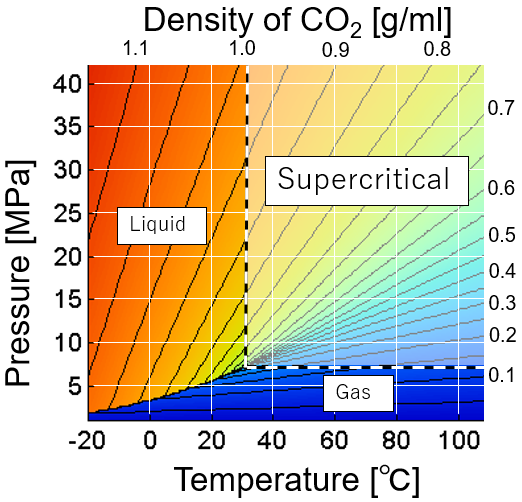
Carbon dioxide is a gas at normal temperature and pressure. When carbon dioxide is cooled, it becomes solid dry ice without passing through the liquid phase at normal pressure. On the other hand, under high pressure, it changes from a gas to a liquid. However, in the region exceeding the critical pressure of 7.38 MPa and the critical temperature of 31.1°C, it becomes supercritical and the boundary between liquid and gas disappears. Supercritical carbon dioxide has the characteristics of both liquid solubility and gas diffusivity. Supercritical carbon dioxide has a low dielectric constant and properties similar to organic solvents, so it is used for purposes such as extraction and cleaning. In addition, physical properties such as solubility can be adjusted by changing temperature and pressure. This is one of the advantages over organic solvent extraction.
What can be done with supercritical CO₂?
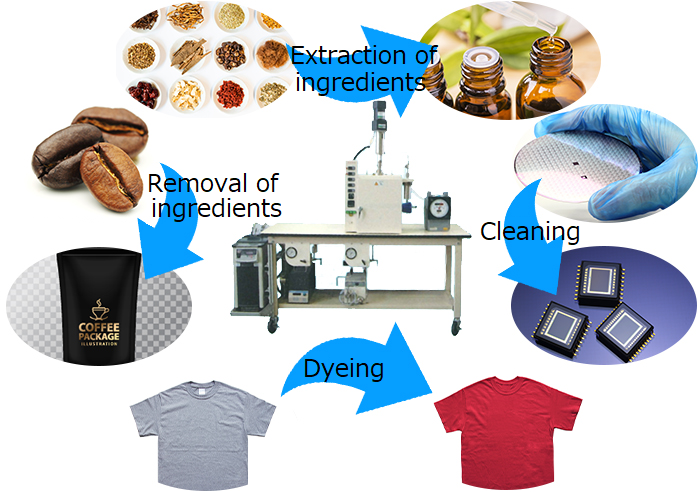
The most common use of supercritical carbon dioxide is extraction. For example, lycopene from tomatoes, astaxanthin from Haematococcus algae, and other active ingredients from various crude drugs. Similarly, it can be used to remove specific components. There are already practical methods for removing caffeine from coffee beans. Applications other than extraction include dry cleaning, which does not require drying. It also has the advantage of preventing breakage due to surface tension when cleaning semiconductor patterns. Furthermore, it is also possible to dye fibers and permeate porous materials such as wood with functional ingredients such as flame retardants.
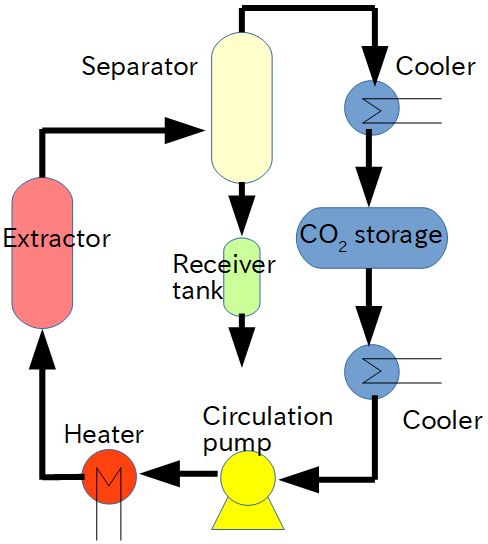
Process flow in mass production
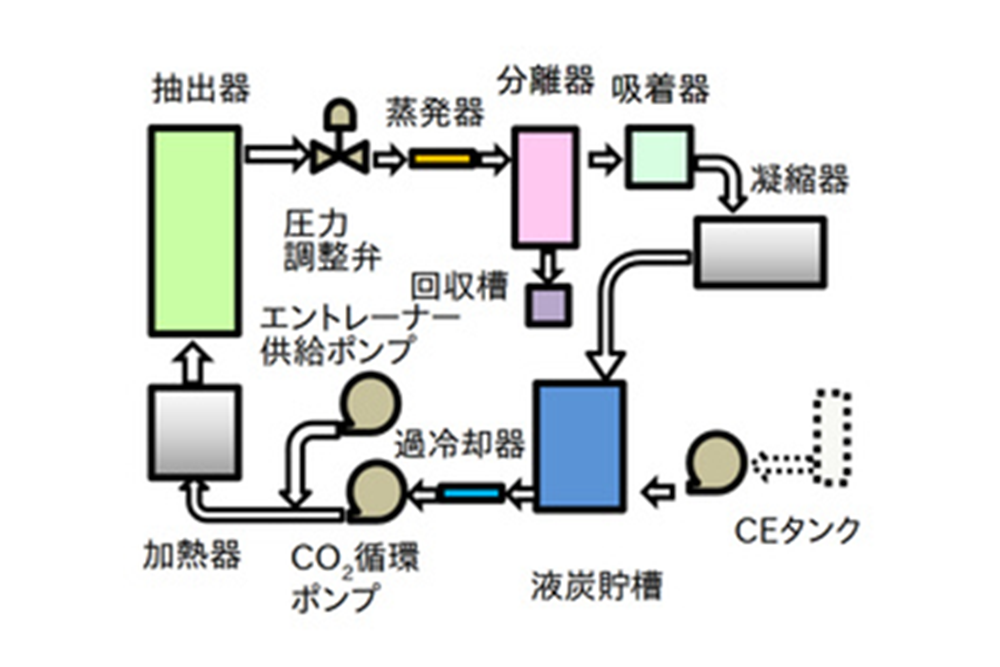
In the trial test, which is performed while changing the test conditions, carbon dioxide gas after use is generally exhausted from the extractor. On the other hand, the process for mass production reuses carbon dioxide. The diagram shows the general flow for extraction. The CO2 is pumped out by the CO2 pump after passing through the cooler from the storage tank. It is then heated to a supercritical state and enters the extractor, which contains raw materials for extraction processing. By continuously exposing the raw material to a stream of supercritical carbon dioxide, the ingredients are gradually dissolved. Entrainers are sometimes added to increase this efficiency. It is then depressurized and enters the separator. At this time, the dissolved components are separated and collected in the collector. The CO2 goes back through the cooler to the storage tank again.
Extraction process by supercritical CO₂
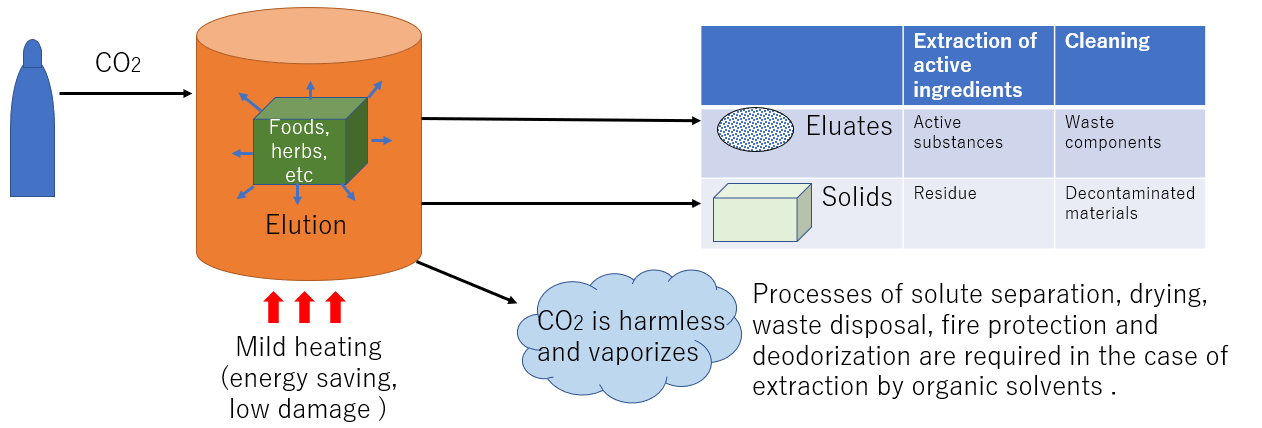
The method of extracting components using an organic solvent requires removal of the organic solvent from the substance after treatment, drying process, waste liquid treatment of the solvent after removal, fire prevention measures, deodorization measures. , and energy for heating is required. Furthermore, energy is required for heating, and high temperatures can cause product deterioration. When supercritical carbon dioxide is used as a solvent, it is removed as a harmless gas after decompression, and the above measures are not required. Therefore, it is applied to a wide range of fields, such as functional foods, medicine, removal of harmful substances, various cleaning and drying, and manufacturing of various substances.