What is supercritical water?
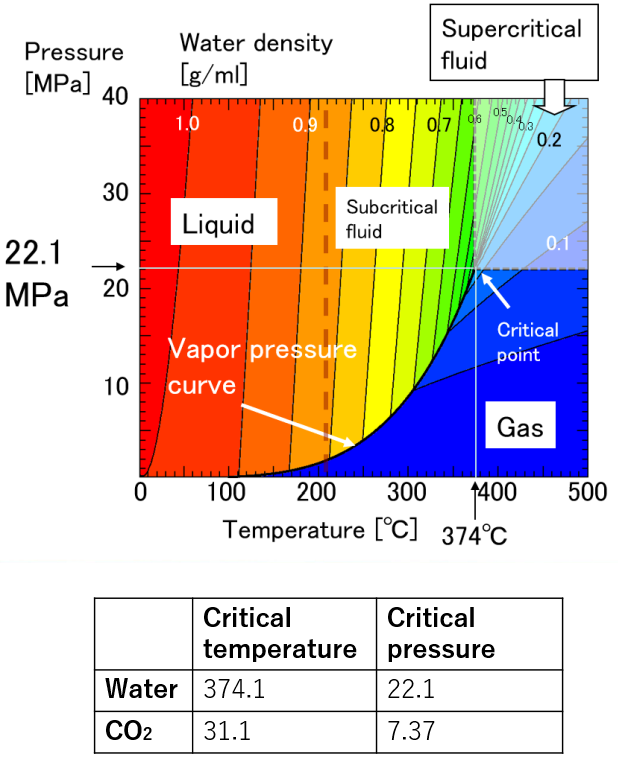
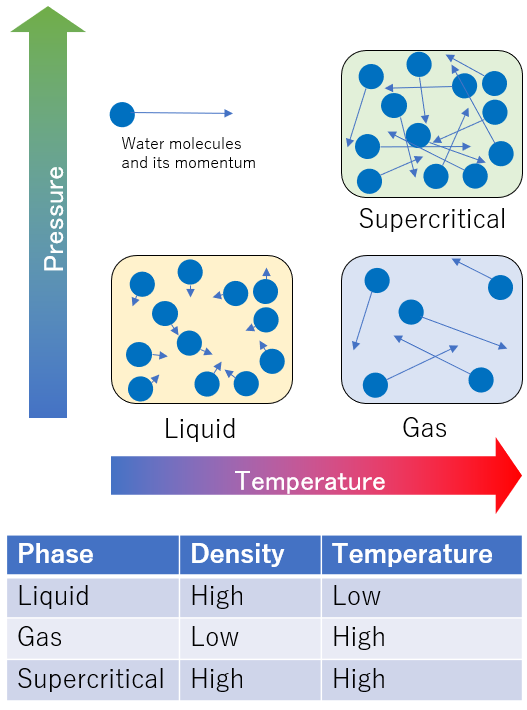
Water has three states: gas, liquid and solid. Here, when the temperature and pressure are increased over the critical point (374°C and 22 MPa), the boundary between liquid and gas disappears. This is called supercritical water (SCW). Supercritical water has a lower density than liquids (much higher than gases) and a lower dielectric constant.
Phase diagram of water
Dynamics of water molecules
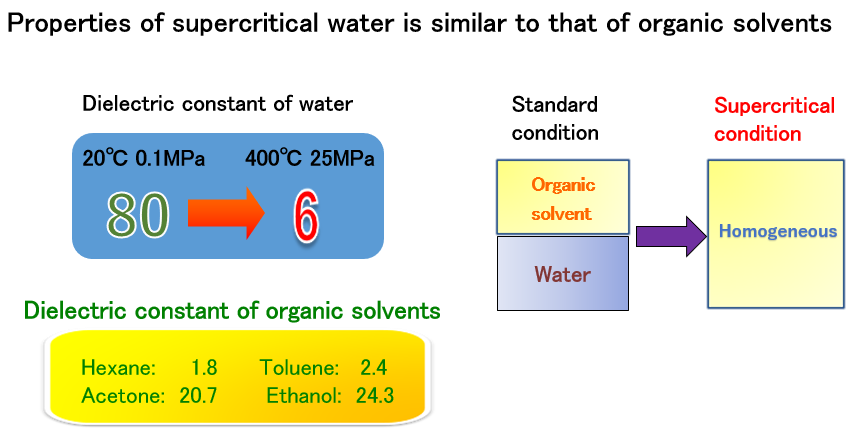
Characteristics of supercritical water
The metal salt aqueous solution used as a raw material for synthesis does not mix with organic solvents in which organic substances are dissolved at room temperature. However, above the water critical point of 374°C and 22 MPa, the dielectric constant of water is greatly reduced and it becomes hydrophobic. Then, the water and the organic solvent that do not mix at room temperature are mixed. This enables the synthesis of organic-inorganic hybrid nanoparticles.
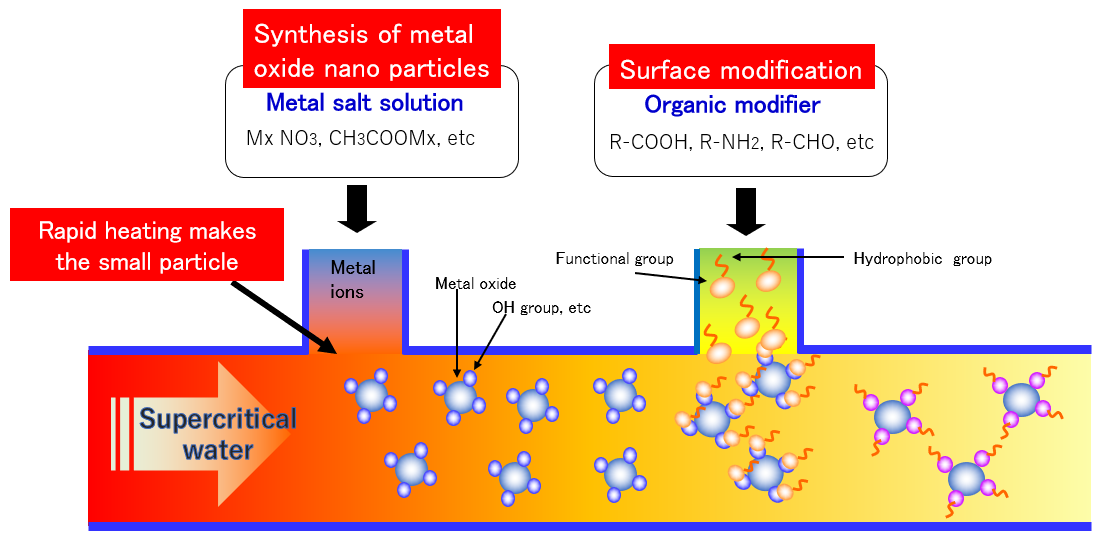
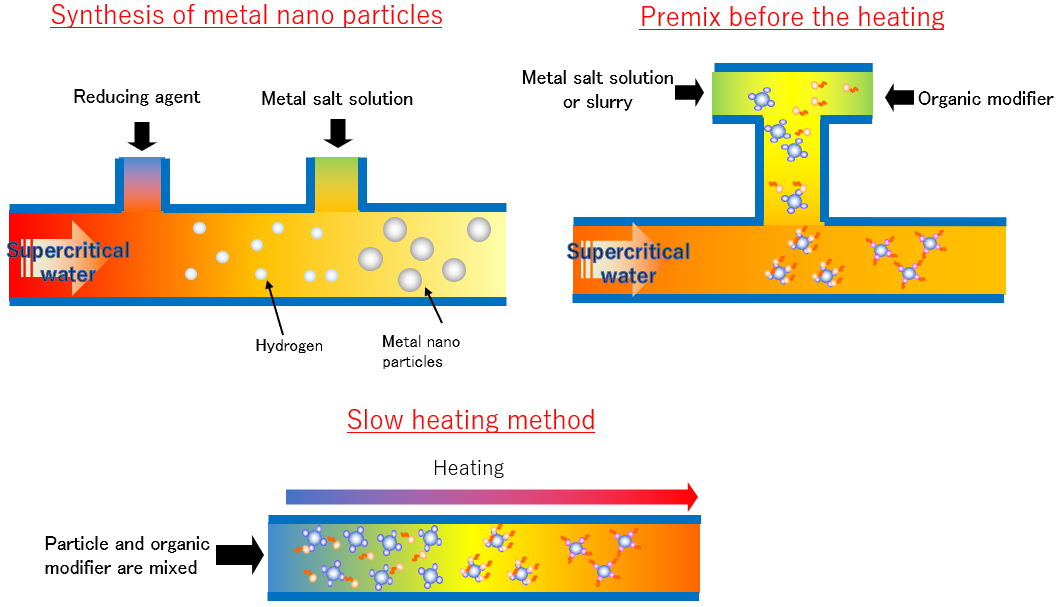
Reaction mechanism of supercritical water nanoparticle synthesis
When a room-temperature metal salt solution is mixed with high-temperature water and becomes supercritical condition, metal ions react with water to form highly crystalline metal oxide nanoparticles. When the nanoparticles are further mixed with an organic modifier solution, it is possible to obtain organic-inorganic hybrid nanoparticles in which the surface of the nanoparticles is covered with organic group.
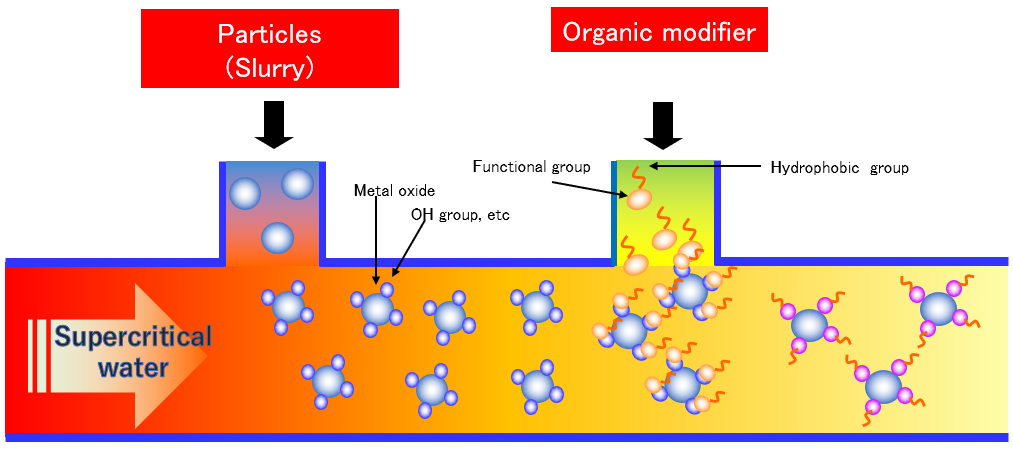
Organic modification of specific particles is available
Our system can be customized. For example, you can change where ingredients are mixed, the order of mixing and heating, or the rate of heating. The reaction time can also be adjusted by changing the length of the reaction tube. Metal nanoparticles can also be synthesized by using a reducing agent. When the piping connection is changed, operation is possible after the leak check is completed.
Several particle can be synthesized